Newsletter
December 22, 2023
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1.409 of 2023 approved the Regulatory Agenda (RA) which covers the priorities for the 2024-2025 biennium.
The Regulatory Agenda is a planning instrument for the normative activities of the Agency. It presents several priority topics to be regulated by ANVISA whilst its validity. Therefore, such Agenda sheds light on the themes do to be improved by regulatory means for the next two years in Brazil.
Social participation alongside the National Sanitary Surveillance System (Sistema Nacional de Vigilância Sanitária – SNVS) was crucial to the construction of the RA 24/25. There were 1.449 contributions received by ANVISA’s Public Consultancy.
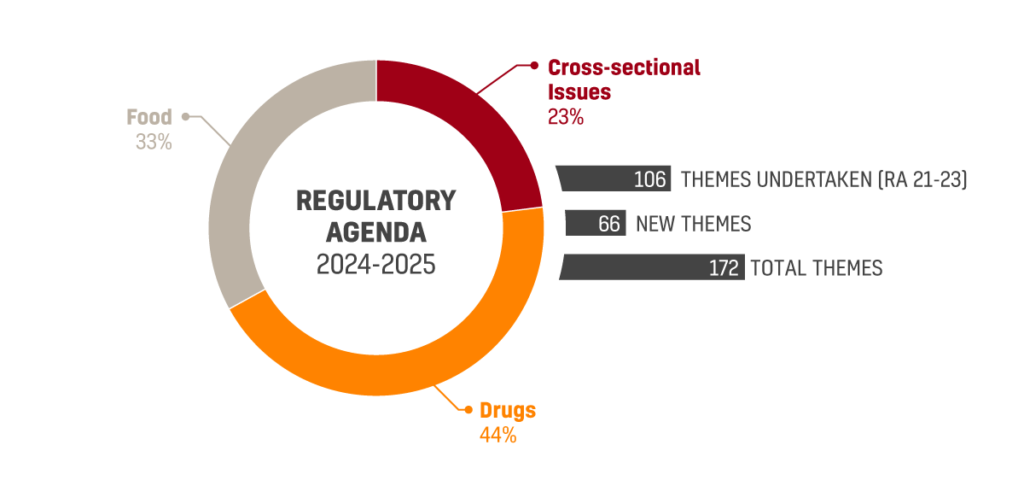
The just approved agenda covers 172 regulatory themes, divided into 16 macro themes. Topics that received most regulatory demands include Drugs with 45 themes, Food with 34 themes and cross-sectional issues with 23 themes.
106 out of the 172 themes pertaining the new agenda are related to the subject matters already announced on the 2021-2023 agenda. 66 themes are new and unrelated to the previous agenda.
The Regulatory Agenda 2024-2025 guarantees visibility and transparency for the normative regulatory activities by ANVISA. It will come into effect on January 2nd, 2024.
For more information regarding ANVISA, please do not hesitate to contact us by email: regulatorio@kasznarleonardos.com.
Last related news
February 4, 2025
BPTO Launches Groundbreaking Study on IP Finance in Brazil
The Brazilian Patent and Trademark Office (BPTO) has released a groundbreaking study on IP Finance in Brazil, exploring the potential of intellectual … BPTO Launches Groundbreaking Study on IP Finance in Brazil

January 28, 2025
International Data Protection Day: Brazilian scenario characterized by Authority’s leading and the rise of lawsuits
On January 28th, we celebrate International Data Protection Day, a significant occasion to reflect on regulatory progress and the challenges faced in the … International Data Protection Day: Brazilian scenario characterized by Authority’s leading and the rise of lawsuits

January 13, 2025
Changes in Meta’s Content Moderation Policies: Repercussions in Brazil
Last Tuesday (January 7th, 2025), Mark Zuckerberg, CEO of Meta, announced on his Instagram profile a change in the content moderation policies … Changes in Meta’s Content Moderation Policies: Repercussions in Brazil




